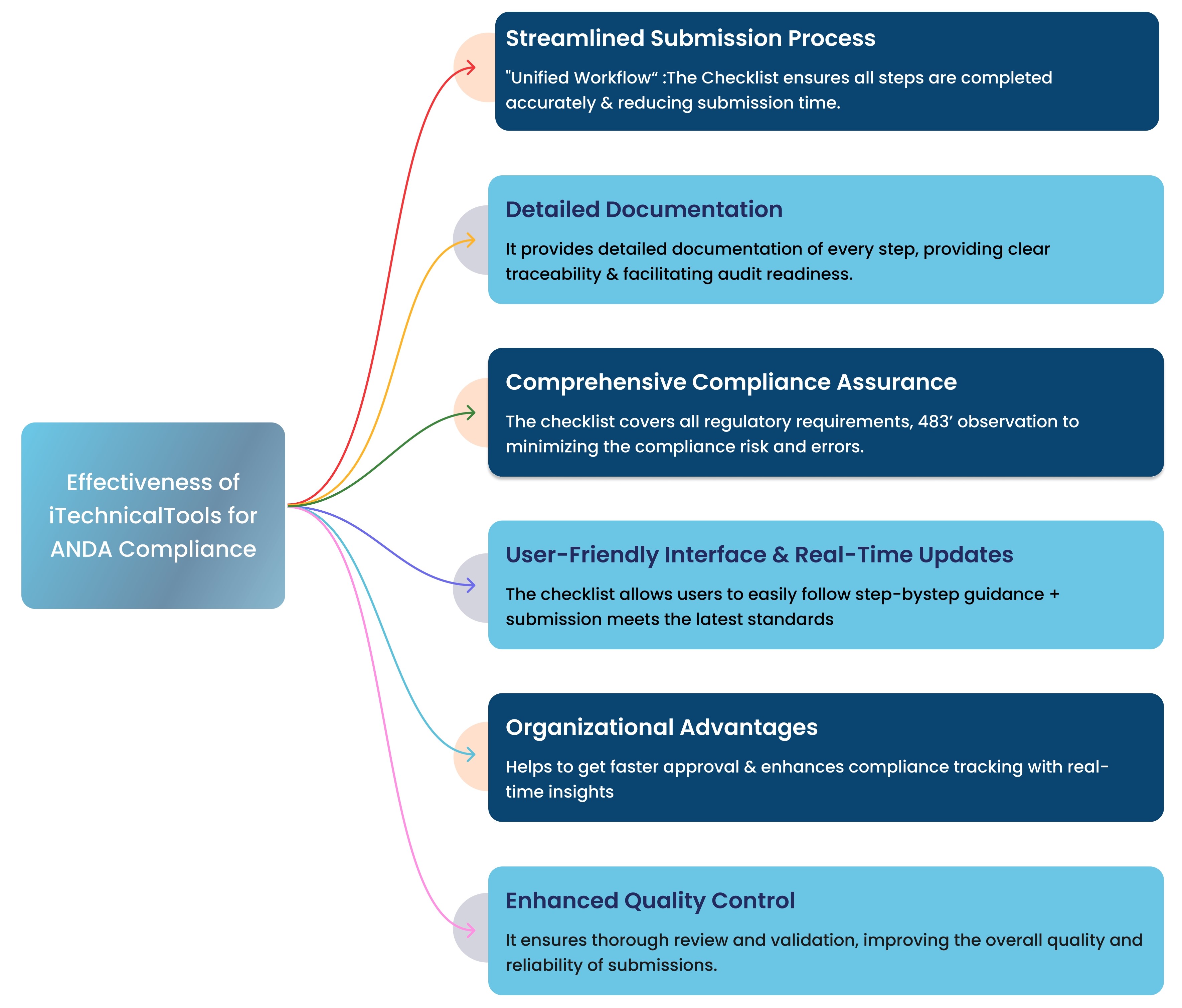
Effectiveness of iTechnicalTools
Innovative AI (Artificial Intelligence) Technical tools to file
quality applications which minimize the deficiencies and “483
database” to minimize the observations & other RA tools.
Regulatory approval procedures are becoming increasingly stringent
and time-consuming. Not having the right regulatory strategy and
Technical tools in place will cost time and money and may result in
the failure or delay of your application, and acceptance and lead to
Major CRs.
These tools help organization to get straight ANDA acceptance, No repeated deficiencies & minimize MAJOR CRs & enhances compliance tracking with real-time insights for improvement.
Increase your program’s chance of expedited success, iCretegy team
provides robust regulatory strategy, consulting, and regulatory
submissions support for your application with customized
iTechnicalTools from filing, amendment, approval to life cycle
management.
This AI based database organizes and structures 12,000+ Form 483 observations. It includes observations from all 6 USFDA cGMP systems: Quality, Production, Facilities & Equipment, Laboratory Controls, Materials, and Packaging & Labelling. It empowers companies to anticipate common inspection risks, strengthen SOPs based on real FDA trends, and benchmark against observations linked to specific inspectors or competitors. Users can search observations by Company name, Investigator name, System type, Year of inspection, Establishment type and Keyword search for terms like Human Error, Data Integrity, OOS, Enviromental monitoring etc. for targeted analysis. Specific observations can be printed or exported as PDFs for internal audits or training. The database also includes 850+ Warning Letters across 845+ companies.
Leverage AI to elevate your regulatory strategy. Our intelligent FDA Deficiency Database analyzes agency feedback - like IRs, DRLs, and CRs - across Oral Solid, Injectable, Ophthalmic, Otic, Nasals, Transdermal, and Other Complex products. It covers key areas such as Specifications, Methods, Validation, Stability, Characterization, Degradation, Manufacturing, Development, Composition, In-process controls, and Process validation. The AI engine provides real-time insights, trend patterns, and predictive analytics to flag potential CMC issues - especially in Drug Substance and Drug Product sections - before submission. It helps reduce review cycles, minimize RTR risk, and accelerate approvals through smarter, data-driven decisions.
Coming soonAnticipate regulatory setbacks before they happen with our AI-driven Complete Response Database. This specialized tool focuses on Major FDA Complete Response (CR) letters across varied dosage forms and product types. By analyzing trends and root causes - whether in CMC or Clinical sections - it helps teams identify and address critical gaps before submission. The goal is fewer CRs, faster approvals, and lower regulatory risk. Avoiding a CR not only saves significant review time but also prevents costly delays in approval and market entry. With intelligent trend detection and actionable analytics, this tool helps you align submissions with FDA expectations more confidently and efficiently.
Coming soon